GE S/5 Datex-Ohmeda Anesthesia Machine (YOM: 2008)
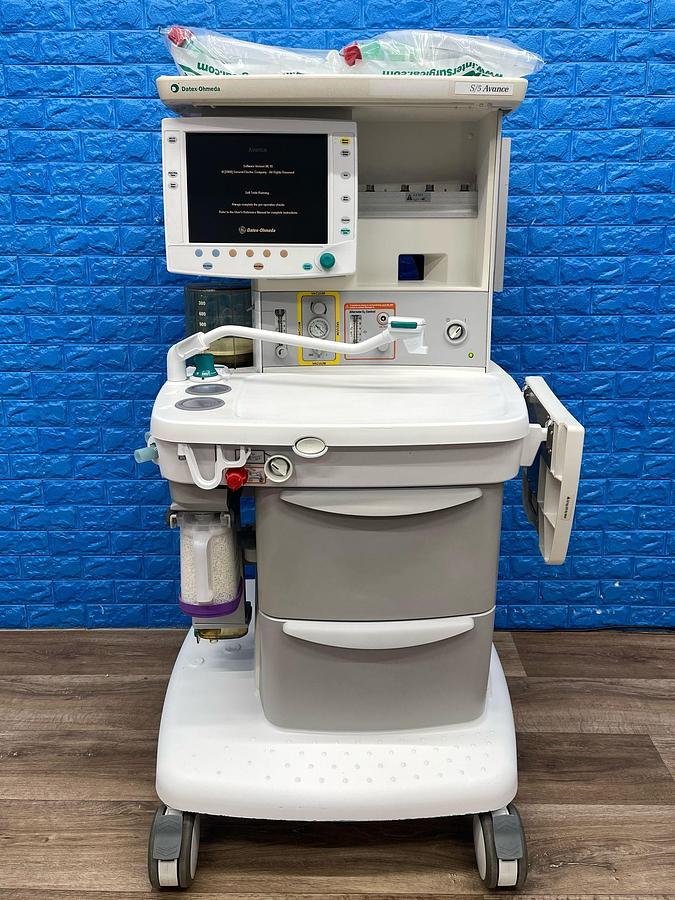
GE S/5 Datex-Ohmeda Anesthesia Machine (YOM: 2008)
Location:Manchester, United Kingdom
or
Call +44 7838577875
Description
GE S/5 Anesthesia Machine for Sale | Flexibility and Reliability
The GE Datex-Ohmeda S/5 Anesthesia Machine, manufactured in 2008, is a versatile and dependable solution for comprehensive patient monitoring and anesthesia delivery. Designed with an open architecture, it seamlessly integrates into various clinical environments, providing clinicians with the tools needed for effective patient care.
Product Specifications:
Why Choose the GE Datex-Ohmeda S/5 Anesthesia Monitor?
Order with Confidence
Looking to purchase the GE Datex-Ohmeda S/5 Anesthesia Monitor? This model is available through reputable medical equipment suppliers, offering quality assurance and support. Please note that monitors and vaporizers are not included at this price; various options are available to suit your specific needs.
We Ship Worldwide
No matter your location, suppliers ensure prompt international shipping to healthcare facilities globally. Receive your GE S/5 Anesthesia Monitor with secure packaging and timely delivery. Order now to enhance your anesthesia monitoring capabilities.
The technical storage or access is strictly necessary for the legitimate purpose of enabling the use of a specific service explicitly requested by the subscriber or user, or for the sole purpose of carrying out the transmission of a communication over an electronic communications network.
The technical storage or access is necessary for the legitimate purpose of storing preferences that are not requested by the subscriber or user.
The technical storage or access that is used exclusively for statistical purposes.
The technical storage or access that is used exclusively for anonymous statistical purposes. Without a subpoena, voluntary compliance on the part of your Internet Service Provider, or additional records from a third party, information stored or retrieved for this purpose alone cannot usually be used to identify you.
The technical storage or access is required to create user profiles to send advertising, or to track the user on a website or across several websites for similar marketing purposes.
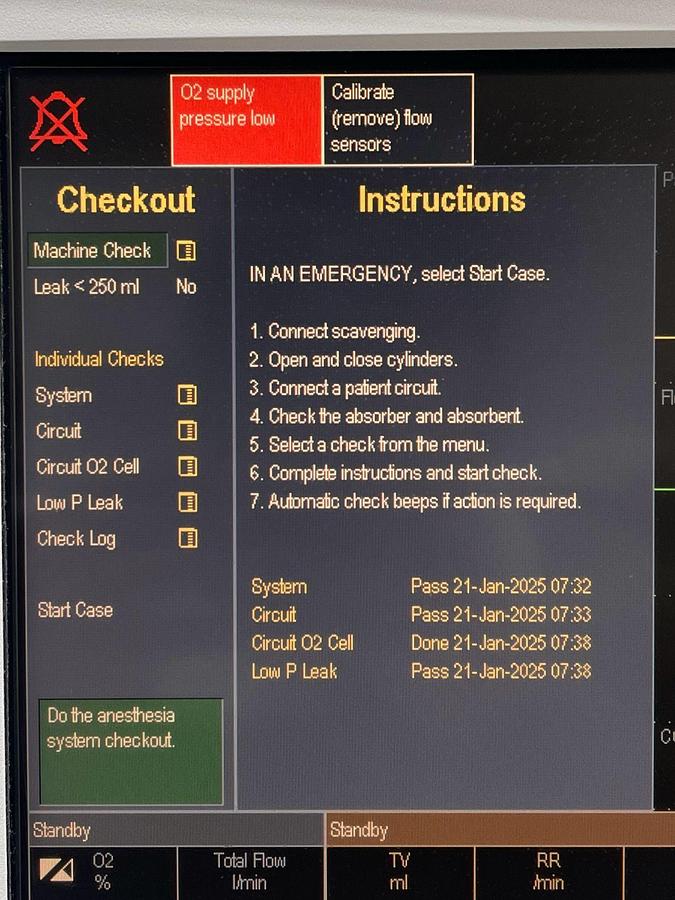
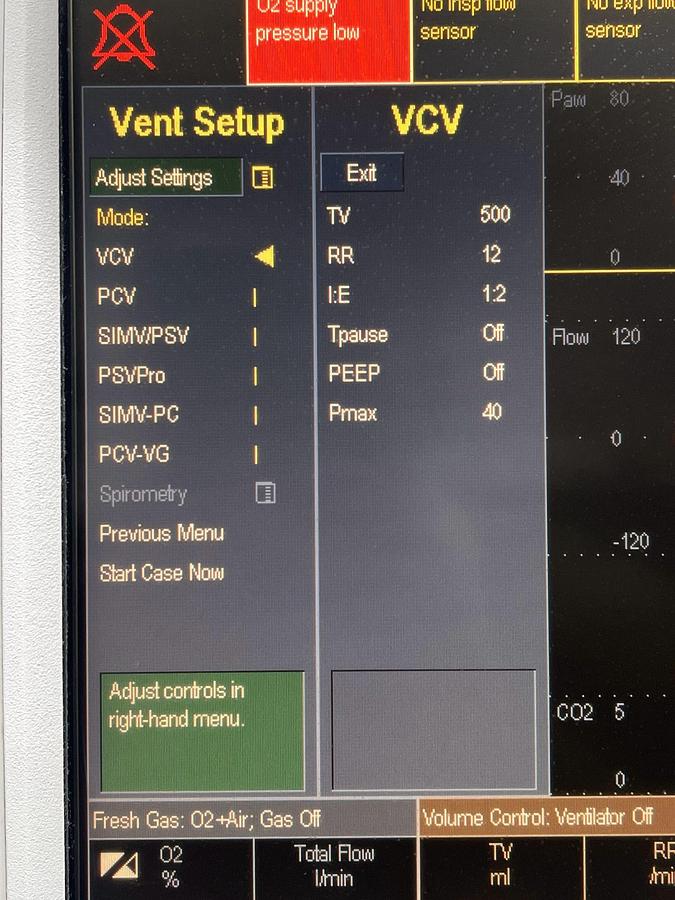
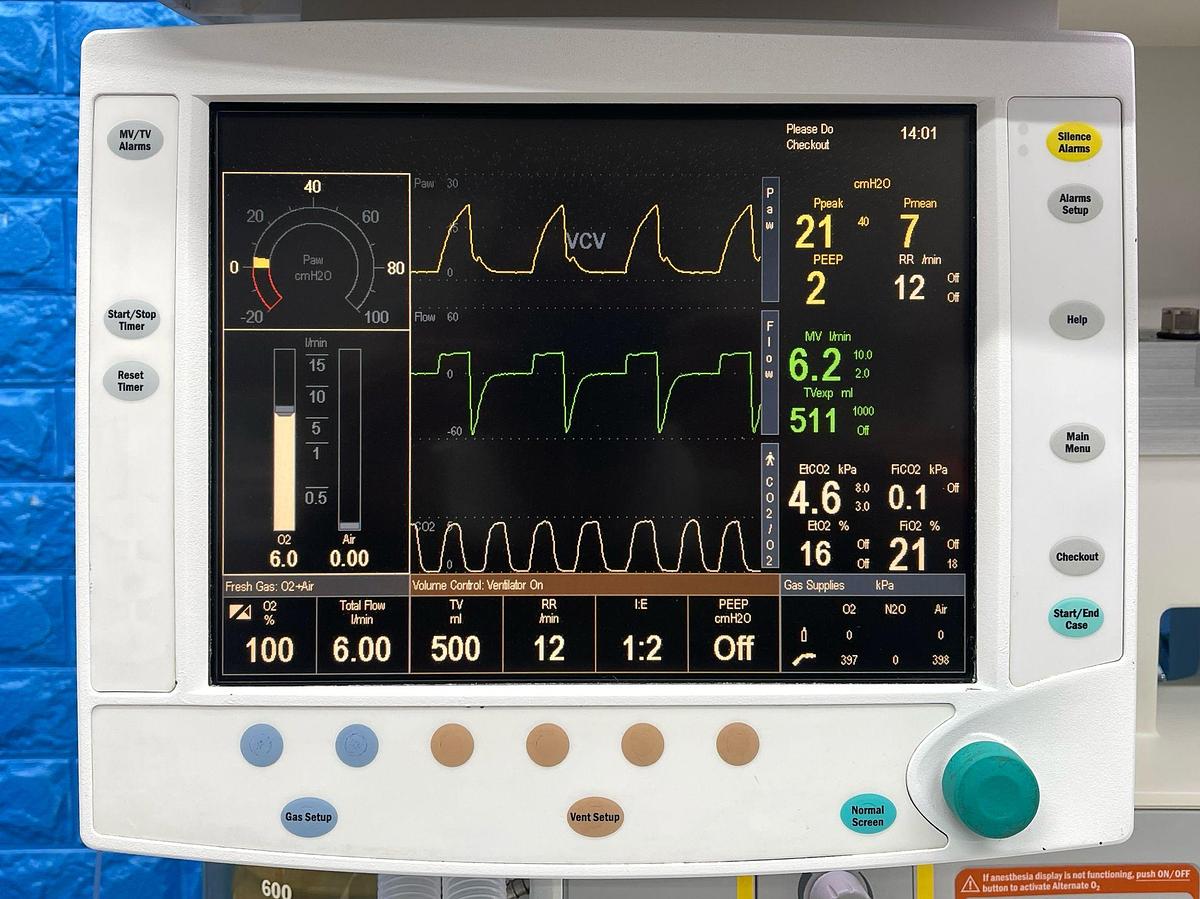
Specifications
| Condition | Used |
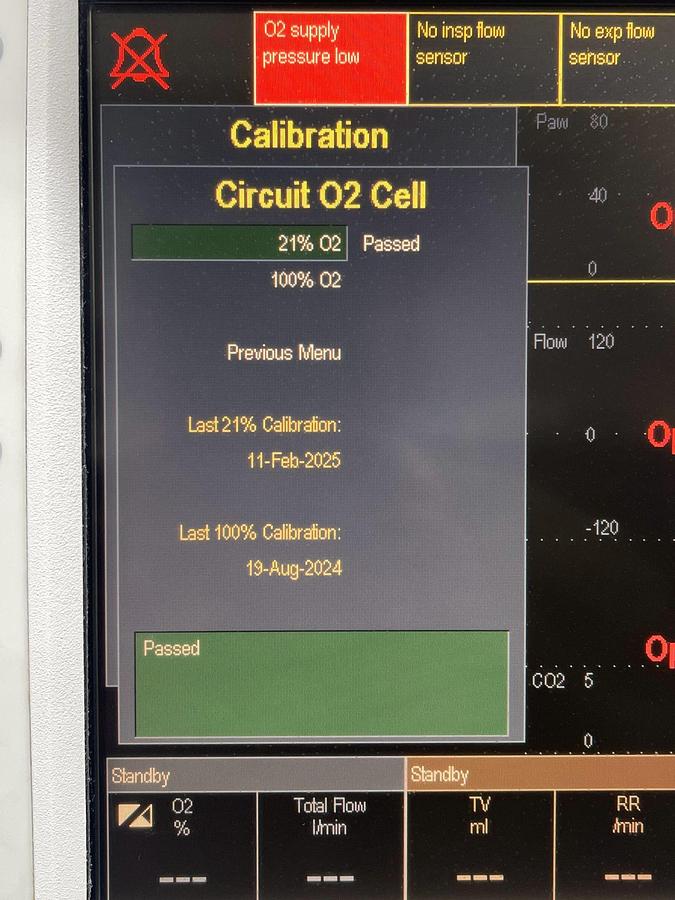
| Monitoring Parameters | ECG, SpO₂, NIBP, Temperature, CO₂, and 5-agent gas analysis |
| Display Options | 12.1″ active matrix color TFT LCD; supports up to 8 waveforms |
| User Interface | Customizable layouts with integrated Command Board and ComWheel for intuitive navigation |
| Alarm System | Anesthesia-dedicated alarms with color and audio coding; automatic limit functions |
| Power Supply | 100 to 240V ±10%, 50/60 Hz; built-in NiMH battery with up to 1.5 hours of operation |
| Comprehensive Monitoring | Offers a full suite of vital sign monitoring, ensuring patient safety during anesthesia. |
| Flexible Configuration | Modular design allows for easy integration with various anesthesia delivery systems. |
| User-Friendly Interface | Customizable displays and intuitive controls streamline workflow for clinicians. |
| Data Management | Advanced trend analysis and reporting capabilities support informed clinical decisions. |
| Durability | Built to withstand the demands of busy operating environments, providing long-term reliability. |